Fighting Cancer and Infectious Diseases


KUPANDO is a pioneering Berlin-based biopharmaceutical company developing TLR 4/7 agonists that stimulate innate immunity.
Our Mission is …
- to provide, not only a safe but also affordable, immunotherapy to cancer patients, and
- to develop immunostimulatory agents with the means to prevent infectious diseases.

TLR 4/7 AGONISTS
Drug Candidates
Kupando develops innovative drug candidates – TLR 4/7 agonists – which target Toll-Like Receptors (TLRs) responsible for the detection of cancer and infection signature molecules, and for the subsequent activation of the innate immune system.
The TLR 4 and TLR7 ligands, which can be produced in a cost-effective fashion, are co-encapsulated in a formulation providing a multitude of options for administration routes and applications.

A multitude of possibilities
Systemic treatment for solid tumors
(tissue agnostic)
Immune-stimulatory agent without antigen
(e.g. prophylactic against future pandemics)
Vaccine adjuvant for universal influenza and others

[click image to enlarge]
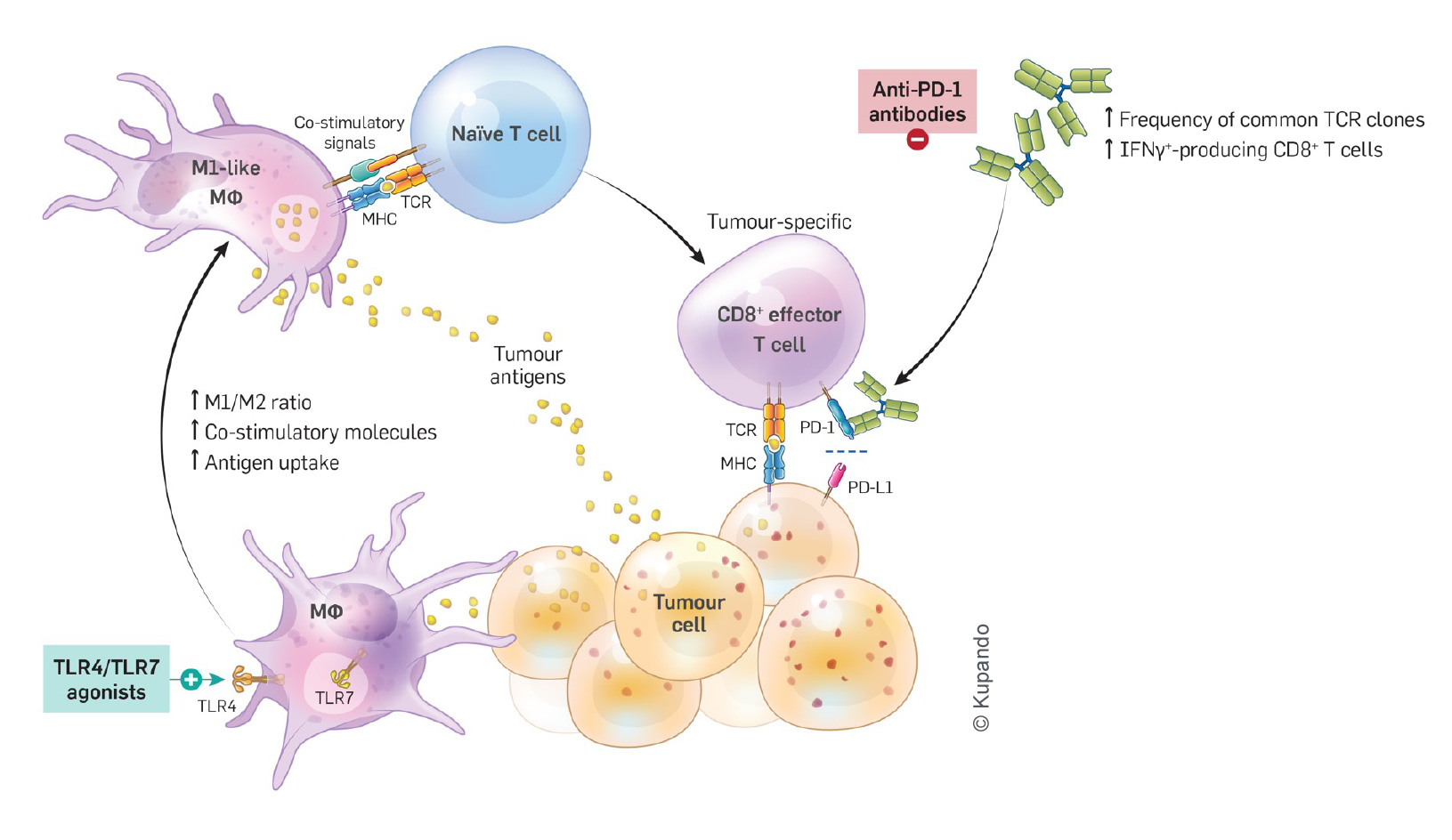
TLR Agonists Activate a Different MoA from Checkpoint Inhibitors — Additive Effect
Single agent or combinations
In oncology, Kupando’s lead candidate KUP101 has the potential to be used as a stand-alone treatment for solid tumors or in combination with other drugs.
In vivo studies have shown a synergistic/additive effect with checkpoint inhibitors.

Advantageous features
- High specificity for target
- No relevant off-targets
- High potency and therefore low dose
- Antigen-sparing effect
- Fast intracellular uptake and therefore improved safety
First-in-class encapsulated small molecules
Kupando’s drug candidates are the only TLR 4/7 agonists in development.
Proof-of-concept data have been confirmed in multiple animal experiments and independent research units. IND-enabling work is ongoing for the treatment of solid tumors and the prevention of infectious diseases.

TLR 4/7 AGONIST
Scientific Background
Vertebrae have two immune systems: (1) an innate immune system and (2) an adaptive one. Innate immunity is inborn. On the contrary, adaptive immunity is acquired over time while developing resistance against pathogens.
From an evolutionary point of view, the innate immune system has evolved over millions of years and has proven to be highly efficacious. It reacts within minutes to days, is not limited by specificity and capable to combat a broad spectrum of pathogens. For decades though, the role and the importance of this part of the immune system have been undervalued and it is now the target of Kupando’s approach.
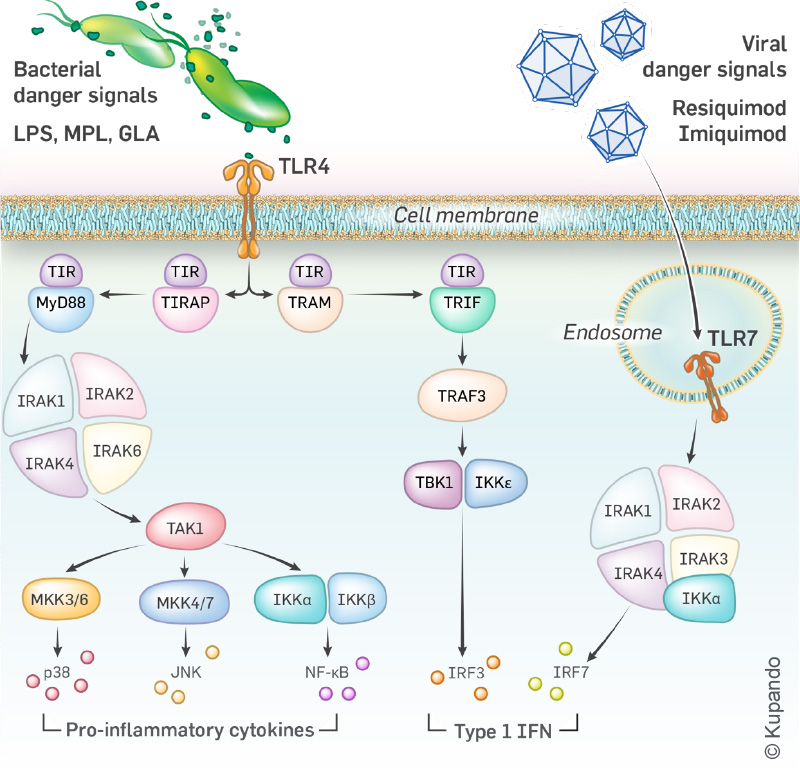
TLR 4/7 agonists stimulate innate immunity by activating Toll Like Receptors (TLRs) and therefore provide a unique tool to fight cancer and infectious diseases.
TLRs operate to detect signature molecules that herald infection and cancer by activating the innate immune system.
There are 10 known TLRs in the human innate immune system. KUP101 specifically stimulates TLR 4 and 7 (TLR 4/7 agonist).
In primates, TLR 7 is expressed mainly by plasmacytoid dendritic cells and B cells. Active TLR 4 is expressed by many more cell types, including endothelial cells and macrophages.
The purpose of an innate immune stimulator is to initiate an adaptive immune response.
If a tumor has few infiltrating dendritic cells and B cells though, a TLR 7 agonist alone may not be a sufficient stimulant. KUP101’s co-encapsulated formulation, which provides additional co-localized TLR 4 agonists, causes local cytokine and chemokine release, especially from the endothelial cells that line the abundant micro vessels that penetrate tumors. In turn, this leads to the recruitment of more dendritic cells and B cells from the blood stream to the tumor, thereby initiating a CD8 cytotoxic immune response.
Both TLR 4 and TLR 7 are validated targets: TLR 4 is the target of MPLA, a vaccine adjuvant. TLR 7 is the target of Imiquimod, a drug used against bladder cancer, actinic keratosis, basal cell carcinoma and HPV-induced epithelial lesions.

TLR 4/7 AGONIST
Publications
Kupando’s drug development is supported by scientific research, see below a short list of scientific publications. For additional information or publications by our team or our collaborators, please talk to us.
October 2022
A Dual Adjuvant System for Intranasal Boosting of Local and Systemic Immunity for Influenza Vaccination
Sato-Kaneko et al.
Vaccines
June 2020
A Novel Synthetic Dual Agonistic Liposomal TLR4/7 Adjuvant Promotes Broad Immune Responses in an Influenza Vaccine With Minimal Reactogenicity
Sato-Kaneko et al.
frontiers in Immunology
June 2018
Induction of oligoclonal CD8 T cell responses against pulmonary metastatic cancer by a phospholipid-conjugated TLR7 agonist
Hosoya et al.
PNAS
Sept 2017
Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer
Sato-Kaneko et al.
JCI Insight
July 2017
Synthetic Toll-Like Receptor 4 (TLR4) and TLR7 Ligands Work Additively via MyD88 To Induce Protective Antiviral Immunity in Mice
Goff et al.
Journal of Virology;
American Society for Microbiology


Our Name
Our company name “Kupando” derives from the Swahili word “kupanda”, which means to mount, to ascend or to rise.
This is what we wish to the patients potentially benefitting from our drug candidate.
This is why we chose the ascending crown crane as our Company Bird. As a messenger of luck and guardian of the health of people and cattle, it is revered in many cultures in the Sahel region south of the Sahara.
The picture of our Company Bird was taken by Johanna Holldack, our CEO, when she was travelling in Kenya and came up with the concept behind Kupando.

Management
Kupando is led by a management team with a wealth of international biotech and pharma experience gained in both Europe and the US in the fields of immunology, infectious diseases and oncology.
With over 100 drug development projects and a huge variety of transactions under its belt, our team brings extensive expertise in business and drug development including in manufacturing, non-clinical and clinical development of vaccines and drugs, as well as regulatory pathways in Europe, the US and Asia.

CEO & Founder
- 30+ years’ experience in drug development:
– Oncology (Proleukin, Eligard, Veregen, TMX-101, etc.)
– Vaccines (Influenza, Rabies, DTaP, etc.) - 4 successful Exits (IPO and trade sales: MediGene, Borean Pharma, Telormedix, Amal Therapeutics)

CFO
- Corporate Finance & Investor Relations
- Managed two IPOs and several M&A transactions
- Worked as top Management Consultant and Start-up Coach
- 20 years’ experience in the Biotech Industry
- Business Economist by training

Head of Regulatory Affairs
- Founder and Managing Director, Regulatory Strategies Consulting
- Senior regulatory advisor, Granzer Consulting
- Global Head of Regulatory Affairs, MediGene AG
- Visiting Scientist, Memorial Sloan Kettering Cancer Center

Strategic Marketing & Business Development
- 30+ years’ experience across pharma, biotech and venture
- Product development strategy, business development, strategic marketing
- Global Head, VP Strategic Marketing at Novo Nordisk, Telormedix
- Venture Partner, Aravis

Head of Operations
- 35+ years’ experience in drug development (Sermino, Cabaser, Reboxetine, Xadago, TMX-101)
- Expert in CMC, Preclinical Development and Toxicology
- VP of Development at Gain Therapeutics listed on NASDAQ
- 2 successful Exits as Co-founder of Telormedix and Head of Development at Inositec

Head of Clinical Operations & Project Management
- 15+ years’ experience in Clinical Operations
- Director Clinical Operations at MiGenTra and MOLOGEN
- Head of Clinical Project Management at Cardior Pharmaceuticals
- Clinical Trial Management
(early phase oncology/hematology)
at NOXXON Pharma
About KUPANDO
Advisory Board
Kupando is backed by an industry approved advisory board.

Chairman
- 30+ years’ experience in the global life science industry (specialty care, vaccines and immunotherapy)
- CEO of MRGN Advisors, Regional Partner of Mérieux Equity Partners and Senior VP International Business at CanSino Biologics, several Biotech board memberships
- Has worked for Sanofi, Aventis, BMS, Schering-Plough and others

Deputy Chairman
- Head of Asset Leadership and member of the R&D Leadership Team at AbbVie (USA)
- Held global R&D leadership positions at Takeda, Shire, Abbott, Astellas, Hoechst Marion Roussel and others
- Has overseen the design and conduct of several key global development programs and successful product registrations

Board member representing Remiges Ventures
- Principal at Remiges Ventures
- Lead and contributed toward many drug discovery projects including oncology and cardiovascular areas
- Has worked for Novatis, Boheringer Ingelheim, Mochida Pharma, RaQualia Pharma

Board member representing a private foundation
- CEO & Founder of LifeCare Partners and kineo finance
- Founded, built and managed BioMedPartners, InterPharmaLInk and worked for McKinsey and Novartis
- Led 80+ investments in life science companies

Board member representing Brandenburg Kapital
- Investment Director Life Sciences, Brandenburg Kapital GmbH
- 15+ years’ industry experience in drug development, diagnostics, and life science tools
- Held BD and M&A positions at Sartorius AG, Thermo Fisher Scientific, and Mologen AG
Scientific Adviser & Inventor

Inventor and Scientific Advisor
- Currently working on adjuvant discovery and vaccine research
- Played key role in founding Dynavax, IDEC, Telormedix, Vical and others
- About 600 papers published
- Professor Emeritus, Moores UCSD Cancer Center
- Member of the National Academy of Science

Press Releases

Kupando raises €13 million in Series A funding round
Schönefeld/Berlin, 26. September 2022 – Kupando, a pioneering biopharmaceutical company developing TLR 4/7 agonists that stimulate innate immunity for use in oncology and infectious diseases, announces today the closing of its Series A funding round which raised €13 million.


Meet us at
INV€$TIVAL SHOWCASE
18 November 2024
London, UK
Johanna Holldack, CEO attending
Angelika Leppert, CFO attending

Contact
Kupando GmbH
Willy-Brandt-Platz 2
12529 Schönefeld
Germany
E: contact@kupando.com
www.kupando.com
Fighting Cancer and Infectious Diseases